Abstract
Background: Mantle cell lymphoma (MCL) is a B cell lymphoma subtype that comprises 6-8% of non-Hodgkin's lymphoma. Bruton's tyrosine kinase (BTK), a crucial component of the B cell receptor (BCR) pathway, is highly expressed in MCL cells. Ibrutinib, a first-in-class oral covalent kinase inhibitor of BTK, was approved by the U.S. Food and Drug Administration for the treatment of relapsed/refractory MCL. Ibrutinib has demonstrated antitumor activity, with an overall response rate of 68% in relapsed/refractory MCL and a median duration of response of 18 months. However, resistance to ibrutinib is a major clinical challenge, prompting an urgent need to identify alternative therapeutic options that would benefit this patient population. The mechanisms underlying ibrutinib resistance are complicated, and studies fully elucidating the intricate networks responsible for therapeutic resistance are still under investigation.
MicroRNAs (miRNAs) are small non-coding RNAs that regulate gene expression by silencing messenger RNA (mRNA) targets. MiRNAs have an important role in cancer development, progression and drug response. To date, ibrutinib resistance was mainly profiled in the coding-genes regions of the genome, while the association between the non-coding regions and ibrutinib resistance is yet to be elucidated in MCL.
Aims: (i) to identify specific miRNA expression profiles distinguishing between ibrutinib-sensitive and -resistant MCL patients and (ii) to identify the mechanism by which miRNAs affect therapeutic efficacy in MCL pathogenesis.
Methods: We created three ibrutinib-resistant cell lines: Jeko_R, Rec_R and Mino_R by gradually increasing ibrutinib dosage over time. miRNA and mRNA expression profiles were determined using small-RNA and RNA next-generation sequencing, respectively. MiRNA/gene and protein expression profiles were validated by qRT-PCR and Western blot analyses in sensitive and resistance cell-lines and patients biopsies (n=20).
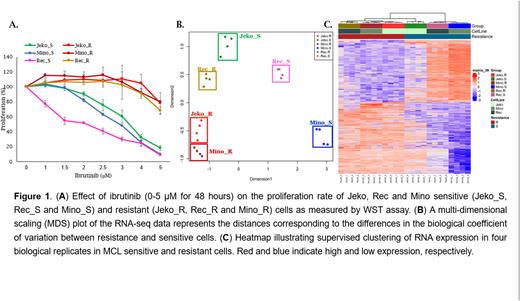
Results: Proliferation assay showed that the IC50 of ibrutinib in parental ibrutinib-sensitive (Jeko_S, Rec_S and Mino_S) cells was 3.5 µM, 1.75 µM and 2 µM respectively, for 48 hours. This concentration had no effect on the proliferation rate of Jeko_R, Rec_R and Mino_R cells (Figure 1A). Moreover, treatment with ibrutinib for 72 and 96 hours, resulted in a significant increase in the levels of apoptosis in the sensitive cell lines, however, did not affect the apoptosis levels of the resistant cells. RNA-sequencing was employed to compare the gene expression profiles in sensitive vs resistant cells. Ibrutinib sensitive cells were clustered separately from the resistant cells (Figure 2B-C). Small-RNA sequencing for miRNA profile showed similar clustring results as mRNA expression pattern. Crossing the miRNAs binding site sequences (seed region) with their mRNA target sequence revealed a potential involvement of miRNAs in regulating pro-cancerous pathways such as MAPK, PI3K-AKT, mTOR, NFkB and mitochondrial energy-related pathways-Oxphos signaling and the TCA cycle in the resistance cell lines. We found a subset of miRNAs that regulate the MAPK-ERK cascade including miRs-221, 146a, 182, 342 and the let-7 family members. All of them were downregulated in ibrutinib resistance MCL patients. Moreover, MCL resistance cells showed over-expression of pERK as comapred to the sensitive cells. Treatment of MCL resistance cell lines with MEK inhibitor (cobimetinib) resulted in significant downregulation of pERK protein level and reduced cell proliferation.
Conclusions: Overall, our data indicates that miRNAs have an important role in drug resistance mechanisms by upregulating MAPK-ERK signaling pathway in ibrutinib resistance MCL patients. This pathway can be targeted by using MEK inhibitors, suggesting novel strategies for combating resistance.
Disclosures
Gomes da Silva:Janssen, Gilead Sciences, MSD, Celgene, Roche, Takeda, Novartis, ADC Therapeutics: Consultancy; Janssen: Speakers Bureau; Gilead Genesis: Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Research Funding. Moita:Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Trněný:Abbvie: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Zentiva: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Research Funding; Gilead Sciencis: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal